#Global In Vitro Toxicity Testing in Chemical Market application
Explore tagged Tumblr posts
Text
Food Allergy Diagnostics And Therapeutics Market Insights: Detailed Overview of Market Size, Share, Projected Growth
The global food allergy diagnostics and therapeutics market size is expected to reach USD 13.28 billion by 2030, registering a CAGR of 8.0% during the forecast period, according to a new report by Grand View Research, Inc. The market is expected to grow in the coming years owing to increasing incidence of food allergies coupled with rising demand for products for management of these conditions.

Food Allergy Diagnostics And Therapeutics Market Report Highlights
Therapeutics segment dominated the market in 2023 due to growing demand for antihistamines and epinephrine auto-injector
Diagnostic segment includes instruments, consumables, and services, wherein consumables expected to grow at the fastest CAGR during the forecast period
The most common tests used to diagnose food allergies are skin prick tests and blood tests
Peanut allergen source segment is anticipated to register lucrative growth over the forecast period, owing to novel product launches coupled with rising incidence of peanut allergies worldwide.
Hospitals & clinics dominated the food allergy diagnostics & therapeutics market in 2023 owing to increase in number of hospitalizations due to severe allergic conditions
For More Details or Sample Copy please visit link @: Food Allergy Diagnostics And Therapeutics Market Report
Medical was the largest application for PEG, accounting for more than 40% of market share in 2022. Superior blending, hygroscopicity, and non-toxic properties of PEG have resulted in high demand for the chemical in numerous pharmaceutical products such as tablets and ointments. Increasing pharmaceutical expenditure, particularly in emerging economies of India, China, and Brazil, is expected to boost PEG market over the forecast period. Growing demand for paints & coatings coupled with the increasing use of PEG as a solvent due to its low VOC emissions is anticipated to fuel market growth over the next six years.
However, administering epinephrine using an auto-injector during the early stage of anaphylaxis might help avoid any serious complications. Treatment for food allergy costs around USD 25 billion annually in the U.S. Development of cost-effective in vitro diagnostic tests for detection of allergy is expected to fuel the demand for diagnostic products.
List of major companies in the Food Allergy Diagnostics And Therapeutics Market
Astellas Pharma, Inc.
Aimmune Therapeutics
Meridian Medical Technologies
ALK-Abelló Ltd
Medeca Pharma AB
bioMeriux SA
Omega Diagnostics Group PLC
HYCOR Biomedical
HOB Biotech Group Corp Ltd.
Cambridge Allergy Ltd
Medeca Pharma AB
For Customized reports or Special Pricing please visit @: Food Allergy Diagnostics And Therapeutics Market Analysis Report
We have segmented the global artificial food allergy diagnostics and therapeutics market based on product, allergen source, end-use, and region.
#FoodAllergyDiagnostics#FoodAllergyTherapeutics#AllergyTesting#AllergyTreatment#HealthcareInnovation#AllergyDiagnostics#FoodAllergyAwareness#Immunotherapy#Biotechnology#InVitroDiagnostics#ClinicalResearch#FoodSafety#AllergyCare#HealthAndWellness#DiagnosticMarket
0 notes
Text
Unveiling Growth Trends and Market Size in the ADME Toxicology Testing Market
Overview and Scope ADME toxicology testing is a set of studies conducted to assess the potential toxicity of a substance or drug candidate based on its absorption, distribution, metabolism, and excretion (ADME) properties. These tests are used in drug development to ensure the well-being of individuals exposed to substances. Sizing and Forecast The adme toxicology testing market size has grown rapidly in recent years. It will grow from $9.3 billion in 2023 to $10.34 billion in 2024 at a compound annual growth rate (CAGR) of 11.2%. The growth in the historic period can be attributed to stringent regulatory requirements, rising drug development activities, growing concerns about drug safety, advancements in in vitro testing methods, increased outsourcing of toxicology studies.. The adme toxicology testing market size is expected to see rapid growth in the next few years. It will grow to $15.77 billion in 2028 at a compound annual growth rate (CAGR) of 11.1%. The growth in the forecast period can be attributed to rapid expansion of biopharmaceuticals, growing emphasis on personalized medicine, emergence of advanced therapies, enhanced predictive toxicology models, global increase in chemical safety testing. To access more details regarding this report, visit the link: https://www.thebusinessresearchcompany.com/report/adme-toxicology-testing-global-market-report Segmentation & Regional Insights The adme toxicology testing market covered in this report is segmented – 1) By Product Type: Instruments, Software Solutions, Assays Systems, Reagents, Other Products 2) By Method: Cellular Assay, Biochemical Assay, In Silica, Ex-vivo 3) By Technology: Cell Culture, High Throughput, Molecular Imaging, OMICS Technology, Other Technologies 4) By Application: Systemic Toxicity, Renal Toxicity, Hepatotoxicity, Neurotoxicity, Other Applications 5) By End-User: Cosmetics And Household Products, Pharmaceutical Industry, Animal Industry, Food Industry, Other End Users North America was the largest region in the ADME toxicology testing market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the adme toxicology testing market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. Intrigued to explore the contents? Secure your hands-on sample copy of the report: https://www.thebusinessresearchcompany.com/sample.aspx?id=11922&type=smp Major Driver Impacting Market Growth The rising demand for novel drugs is expected to propel the growth of the ADME toxicology testing market going forward. Novel drugs are pharmaceutical substances that have not been previously approved or marketed for therapeutic use. ADME toxicity testing is a major step in drug development to ensure that the novel drug is safe and doesn’t produce any harmful toxins in the body. Key Industry Players Major companies operating in the adme toxicology testing market report are AbbVie Inc., Thermo Fisher Scientific Inc., Boehringer Ingelheim GmbH, Laboratory Corporation of America Holdings, Corning Incorporated, Eurofins Scientific SE, Agilent Technologies Inc., Lonza Group AG, Dassault Systèmes SE, Catalent Inc., Charles River Laboratories International Inc., Beckman Coulter Inc., PerkinElmer Inc., Bio-Rad Laboratories Inc. The adme toxicology testing market report table of contents includes: 1. Executive Summary
2. Market Characteristics
3. Market Trends And Strategies
4. Impact Of COVID-19
5. Market Size And Growth
6. Segmentation
7. Regional And Country Analysis .
.
.
27. Competitive Landscape And Company Profiles
28. Key Mergers And Acquisitions
29. Future Outlook and Potential Analysis Contact Us: The Business Research Company Europe: +44 207 1930 708 Asia: +91 88972 63534 Americas: +1 315 623 0293 Email: [email protected] Follow Us On: LinkedIn: https://in.linkedin.com/company/the-business-research-company Twitter: https://twitter.com/tbrc_info Facebook: https://www.facebook.com/TheBusinessResearchCompany YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ Blog: https://blog.tbrc.info/ Healthcare Blog: https://healthcareresearchreports.com/ Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
Cell Culture Media And Reagents Market Insights: Comprehensive Global Forecast (2023-2032)

The global market for cell culture media and reagents is expected to develop at a compound annual growth rate (CAGR) of 8.20% between 2024 and 2032, from its estimated USD 6525.2 million in 2023 to USD 13262.92 million in 2032.
The cell culture media and reagents market is experiencing significant growth, fueled by the expanding applications of cell culture techniques in biotechnology, pharmaceuticals, and medical research. Cell culture media and reagents are essential for growing and maintaining cells in vitro, providing the necessary nutrients, hormones, and growth factors to support cellular functions. The rising demand for biopharmaceuticals, including monoclonal antibodies, vaccines, and recombinant proteins, is a primary driver of market expansion, as these products rely heavily on cell culture processes for production. Additionally, advancements in regenerative medicine and tissue engineering, which utilize cell culture for developing novel therapies and artificial organs, are further propelling market growth.
The market is also benefiting from the increasing focus on precision medicine and personalized therapies, where cell culture techniques are employed to develop treatments tailored to individual patient profiles. The growing prevalence of chronic diseases and the need for innovative treatment options are driving research and development activities, leading to a higher demand for high-quality cell culture media and reagents. Moreover, the rise of biobanks and the growing practice of cell-based assays in drug discovery and toxicity testing are contributing to market growth.
Technological advancements in cell culture systems, such as the development of serum-free, chemically defined, and specialty media, are enhancing the efficiency and reproducibility of cell culture processes. These innovations are particularly important in reducing variability and improving the scalability of cell culture operations, making them more suitable for industrial applications. Additionally, the integration of automation and high-throughput screening technologies in cell culture workflows is streamlining processes and reducing manual intervention, further boosting market growth.
The cell culture media and reagents market dynamics are shaped by various factors that influence its growth, trends, and challenges. These dynamics include drivers, restraints, opportunities, and trends that collectively impact the market’s trajectory.
Market Drivers
Rising Demand for Biopharmaceuticals: The increasing need for biopharmaceuticals, such as monoclonal antibodies, vaccines, and recombinant proteins, is a significant driver. These products are heavily reliant on cell culture processes, boosting the demand for cell culture media and reagents.
Advancements in Biotechnology and Medical Research: Continuous innovations and advancements in biotechnology, including regenerative medicine, tissue engineering, and gene therapy, are expanding the applications of cell culture. This drives the need for specialized media and reagents that support these advanced research areas.
Growth in Research and Development Activities: The growing focus on R&D in the pharmaceutical and biotechnology sectors, driven by the need for innovative treatments and therapies, is propelling the market. Increased funding and investment in life sciences research further support this growth.
Precision Medicine and Personalized Therapies: The shift towards precision medicine, which tailors treatments to individual patient profiles, relies on cell culture techniques for developing personalized therapies. This trend significantly increases the demand for high-quality cell culture media and reagents.
Market Restraints
High Cost of Advanced Media and Reagents: The cost of advanced cell culture media and reagents, especially serum-free, chemically defined, and specialty media, can be prohibitively high for smaller research institutions and companies, limiting market accessibility.
Technical Complexity and Expertise Requirement: The complexity of cell culture processes requires specialized knowledge and expertise. This can be a barrier to adoption, particularly in regions or organizations with limited access to skilled personnel and training resources.
Variability and Consistency Issues: Ensuring the consistency and quality of cell culture products is crucial. Variability in media and reagents can impact research outcomes and product efficacy, posing a challenge for researchers and manufacturers.
Market Opportunities
Emerging Markets: The growing biotechnology and pharmaceutical sectors in emerging markets, particularly in the Asia-Pacific region, offer significant growth opportunities. Increasing healthcare expenditure and investments in life sciences research in countries like China, India, and Japan are driving market expansion.
Technological Innovations: Advancements in cell culture technologies, such as 3D cell culture, organ-on-a-chip systems, and automation, present opportunities for market growth. These technologies enhance the efficiency, scalability, and reproducibility of cell culture processes.
Market Trends
Shift Towards Serum-Free and Chemically Defined Media: There is a growing trend towards the use of serum-free and chemically defined media, which offer better control over experimental conditions and reduce the risk of contamination. These media types are becoming increasingly popular in biopharmaceutical production and research.
Integration of Automation and High-Throughput Screening: The integration of automation and high-throughput screening technologies in cell culture workflows is streamlining processes and reducing manual intervention. This trend enhances productivity and efficiency in research and production environments.
Focus on Sustainable and Ethical Practices: There is an increasing focus on sustainable and ethical practices in cell culture, including the reduction of animal-derived components and the use of eco-friendly materials. This trend aligns with broader industry movements towards sustainability and corporate social responsibility.
Key Players:
Sartorius AG
Danaher Corporation
Merck KGaA
Thermo Fisher Scientific, Inc.
FUJIFILM Corporation
Lonza
BD
STEMCELL Technologies
Cell Biologics, Inc.
PromoCell GmbH
More About Report- https://www.credenceresearch.com/report/cell-culture-media-and-reagents-market
The future of the cell culture media and reagents market is poised for dynamic growth, influenced by several emerging trends. These trends are driven by technological advancements, evolving research needs, and increasing applications in various fields of biotechnology and medicine.
1. Growth of 3D Cell Culture and Organoids
The shift from traditional 2D cell cultures to more complex 3D cell cultures and organoids is gaining momentum. 3D cultures provide a more accurate representation of in vivo conditions, enhancing the relevance of research outcomes in drug discovery, cancer research, and tissue engineering. The development and adoption of specialized media and reagents tailored for 3D cell cultures are expected to rise significantly.
2. Expansion of Stem Cell Research
Stem cell research is rapidly expanding, with increasing applications in regenerative medicine, disease modeling, and drug screening. The demand for high-quality, defined media and reagents that support the growth, differentiation, and maintenance of stem cells is anticipated to grow. Innovations in this area will focus on optimizing conditions for various stem cell types, including induced pluripotent stem cells (iPSCs) and mesenchymal stem cells (MSCs).
3. Advancements in Personalized Medicine
The move towards personalized medicine and precision therapies will drive the need for customized cell culture solutions. Media and reagents will need to be tailored to support patient-specific cell lines and treatments. This trend is expected to enhance the development of personalized therapies for a range of diseases, including cancer and genetic disorders.
4. Integration of Artificial Intelligence (AI) and Machine Learning (ML)
AI and ML are becoming integral to optimizing cell culture processes. These technologies can analyze large datasets to identify optimal culture conditions, predict cell growth patterns, and automate routine tasks. The integration of AI/ML will lead to more efficient and reproducible cell culture practices, improving the scalability and reliability of bioproduction.
5. Adoption of Automation and High-Throughput Technologies
The adoption of automation and high-throughput screening (HTS) technologies in cell culture workflows is expected to increase. Automation reduces human error, enhances reproducibility, and allows for the handling of large-scale experiments. High-throughput technologies enable rapid screening of numerous conditions, accelerating research and development timelines.
6. Focus on Sustainable and Ethical Practices
Sustainability and ethical considerations are becoming increasingly important in the cell culture media and reagents market. The move towards reducing the use of animal-derived components and adopting eco-friendly production processes is gaining traction. This trend aligns with broader industry efforts to promote sustainability and corporate social responsibility.
7. Development of Serum-Free and Chemically Defined Media
The industry is moving towards the development and adoption of serum-free and chemically defined media. These media types reduce variability, enhance reproducibility, and mitigate the risk of contamination from undefined components. The shift towards these advanced media formulations is particularly significant in biopharmaceutical production and clinical research.
8. Expansion in Emerging Markets
Emerging markets, particularly in Asia-Pacific, are expected to witness rapid growth in the cell culture media and reagents sector. Increased healthcare expenditure, expanding biopharmaceutical industries, and growing investments in life sciences research in countries like China, India, and South Korea will drive market expansion in these regions.
9. Innovations in Gene and Cell Therapy
The advancements in gene and cell therapy are driving demand for specialized media and reagents that support the production and manipulation of genetically modified cells. As these therapies progress towards commercialization, there will be a growing need for scalable and reproducible cell culture solutions.
Segmentation
By Type of Media
Serum-based Media
Serum-free Media
Chemically Defined Media
Specialty Media
By Format
Liquid Media
Powdered Media
Concentrated Media
By Reagent
Supplements
Cytokines and Growth Factors
Antibiotics and Antimycotics
Buffer Solutions
Enzymes
Stains and Dyes
Browse the full report – https://www.credenceresearch.com/report/cell-culture-media-and-reagents-market
Browse Our Blog: https://www.linkedin.com/pulse/cell-culture-media-reagents-market-aqevf
Contact Us:
Phone: +91 6232 49 3207
Email: [email protected]
Website: https://www.credenceresearch.com
0 notes
Text
Advancing Research: Harnessing the Potential of Cell Culture Media
The Cell Culture Media Market is a critical segment within the biotechnology and pharmaceutical industries, providing essential formulations for the growth, maintenance, and proliferation of cells in vitro. Cell culture media are nutrient-rich solutions containing amino acids, vitamins, minerals, growth factors, and other supplements necessary for cell survival and growth. This market analysis explores the key drivers, trends, challenges, and opportunities shaping the Cell Culture Media Market.
𝐆𝐞𝐭 𝐟𝐫𝐞𝐞 𝐒𝐚𝐦𝐩𝐥𝐞: https://www.marketdigits.com/request/sample/3943
One of the primary drivers of the Cell Culture Media Market is the increasing demand for cell-based technologies in drug discovery, biopharmaceutical production, regenerative medicine, and basic research. Cell culture techniques are essential tools for studying cellular behavior, modeling diseases, screening drug candidates, and producing biologics, such as monoclonal antibodies, vaccines, and cell therapies. As the pharmaceutical industry shifts towards biologics and personalized medicine, there is a growing need for high-quality cell culture media to support these applications.
The Cell Culture Media Market is valued at USD 3.3 billion in 2024 and projected to reach USD 5.6 billion by 2030, with a Compound Annual Growth Rate (CAGR) of 6.8% during the forecast period spanning 2024-2032.
Moreover, advancements in cell culture technology, tissue engineering, and stem cell research have expanded the scope and complexity of cell culture media formulations. Cell culture media are customized to meet the specific requirements of different cell types, including mammalian cells, insect cells, plant cells, and microbial cells. Specialty media formulations, such as serum-free media, chemically defined media, and xeno-free media, offer improved consistency, reproducibility, and performance compared to traditional serum-containing media, addressing concerns related to variability, animal-derived components, and regulatory compliance.
Major vendors in the global cell culture media market are Becton Dickinson and Company, Bio-Rad Laboratories, Inc, Caisson Laboratories Inc., Cell Culture Technologies LLC, Corning , Cytiva, FORTUNE Media IP, FUJIFILM, Hi Media Laboratories Pvt. Ltd., Lonza Group AG, Merck KGaA, PL BioScience GmbH, Pricella Biotchnology Co., Ltd, Thermo Fisher Scientific, Inc. and Others.
Furthermore, the growing adoption of 3D cell culture and organoid culture techniques has fueled demand for specialized cell culture media optimized for three-dimensional cell growth and tissue engineering applications. 3D cell culture models better recapitulate the complex microenvironment and physiological conditions found in vivo, offering more physiologically relevant platforms for drug screening, toxicity testing, and disease modeling. Cell culture media formulations tailored for 3D culture support the growth, differentiation, and functionality of cells in three-dimensional structures, enabling researchers to study complex biological processes and develop more predictive preclinical models.
In addition to traditional research and development applications, the Cell Culture Media Market is witnessing increased demand from the biopharmaceutical industry for large-scale cell culture media formulations used in bioproduction processes. Cell culture media are essential components of bioreactor systems used to produce recombinant proteins, monoclonal antibodies, viral vectors, and cell-based therapies in biomanufacturing facilities. Optimized media formulations, supplemented with growth factors, cytokines, and nutrients, support high-density cell growth, protein expression, and bioprocess efficiency, leading to increased yields, reduced manufacturing costs, and faster time-to-market for biopharmaceutical products.
However, the Cell Culture Media Market also faces challenges and limitations that may impact its growth and adoption. One of the main challenges is the complexity and variability of cell culture media formulations, which can affect reproducibility, scalability, and regulatory compliance. Cell culture media components, such as serum, growth factors, and supplements, may vary in composition, quality, and performance, leading to batch-to-batch variability and inconsistency in cell culture outcomes. Standardization, quality control, and characterization of cell culture media components are essential for ensuring product consistency, reliability, and safety in research and manufacturing settings.
Moreover, the Cell Culture Media Market is subject to regulatory scrutiny and quality assurance requirements to ensure the safety, efficacy, and purity of cell culture media products. Regulatory agencies, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), provide guidelines and regulations for the development, manufacturing, and quality control of cell culture media used in clinical applications and biopharmaceutical production. Compliance with Good Manufacturing Practices (GMP), quality management systems, and documentation requirements is essential for obtaining regulatory approval and maintaining product quality and integrity.
In conclusion, the Cell Culture Media Market plays a crucial role in supporting cell-based research, biopharmaceutical development, and biomanufacturing processes across diverse industries. Despite challenges such as variability, regulatory compliance, and quality assurance, the market continues to evolve with advancements in cell culture technology, media formulations, and manufacturing processes. By addressing unmet needs, improving product quality, and fostering innovation, the Cell Culture Media Market can drive progress and enable breakthroughs in biomedical research, drug discovery, and therapeutic development.
0 notes
Text
Artificial Tissue Market: A Comprehensive Overview of Current Trends and Future Prospects
According to the study by Next Move Strategy Consulting, the global Artificial Tissue Market size is predicted to reach USD 29.83 billion with a CAGR of 12.3% by 2030.
Request a FREE sample, here: https://www.nextmsc.com/artificial-tissue-market/request-sample

In recent years, the field of regenerative medicine has witnessed remarkable advancements, particularly in the development of artificial tissues. These engineered tissues hold immense potential to revolutionize healthcare by offering solutions for tissue repair, replacement, and regeneration. The artificial tissue market is poised for significant growth, driven by evolving technologies, increasing prevalence of chronic diseases, and growing demand for personalized medicine. This article provides a comprehensive overview of the current trends and prospects shaping the artificial tissue market landscape.
Current Trends in the Artificial Tissue Market
Bioprinting Technology Advances in 3D bioprinting technology have transformed the landscape of tissue engineering. Bioprinters can precisely deposit biomaterials and living cells layer by layer to create complex tissue structures. Bioinks, composed of cells and biomaterials, serve as the building blocks for constructing artificial tissues. Researchers have successfully bioprinted tissues such as skin, cartilage, and blood vessels, paving the way for applications in wound healing, organ transplantation, and drug testing.
Biomaterial Innovations Biomaterials play a crucial role in providing structural support and cues for cell growth and tissue regeneration in artificial tissue engineering. Researchers are exploring novel biomaterials with enhanced biocompatibility, mechanical properties, and bioactivity to improve tissue scaffolds' performance. Hydrogels, decellularized matrices, and synthetic polymers are among the biomaterials utilized in artificial tissue fabrication. Surface modification techniques, such as chemical functionalization and electrospinning, enable the customization of biomaterial properties to suit specific tissue engineering applications.
Stem Cell Therapies Stem cells hold immense promise in tissue regeneration and repair due to their ability to differentiate into various cell types. Researchers are exploring the integration of stem cell-based therapies with artificial tissue constructs to enhance tissue regeneration outcomes. Mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), and embryonic stem cells (ESCs) are among the cell types utilized in artificial tissue engineering. Stem cell-derived tissues offer potential treatments for conditions such as cardiovascular diseases, neurodegenerative disorders, and musculoskeletal injuries.
Organ-on-a-Chip Platforms Organ-on-a-chip technologies replicate the physiological microenvironment of human organs, enabling researchers to study organ-level functions in vitro. These microfluidic devices incorporate cells, biomaterials, and microengineering techniques to mimic organ structure and function accurately. Organ-on-a-chip platforms offer insights into disease mechanisms, drug responses, and toxicity testing, facilitating drug discovery and development processes. Liver-on-a-chip, lung-on-a-chip, and heart-on-a-chip models are among the organ-specific platforms used for drug screening and disease modelling applications.
Regulatory Landscape Regulatory agencies play a crucial role in ensuring the safety, efficacy, and quality of artificial tissue-based therapies. Harmonized regulatory frameworks are essential to streamline the development, evaluation, and commercialization of artificial tissue products. Regulatory guidelines provide requirements for preclinical testing, clinical trials, and manufacturing practices to ensure compliance with safety and ethical standards. Collaboration between regulatory agencies, industry stakeholders, and academic researchers is necessary to address regulatory challenges and facilitate the translation of artificial tissue innovations into clinical applications.
Future Prospects and Opportunities
Personalized Medicine The integration of artificial tissue technologies with patient-specific data holds promise for personalized medicine approaches. Advances in omics technologies, such as genomics, proteomics, and metabolomics, enable the characterization of individual patients' biological profiles. Combined with tissue engineering techniques, personalized tissue constructs can be tailored to match patients' unique anatomical and physiological characteristics. Patient-specific tissues offer potential treatments for conditions such as congenital defects, traumatic injuries, and degenerative diseases.
Disease Modelling Artificial tissues provide valuable platforms for modelling complex diseases and studying disease mechanisms in vitro. Patient-derived tissue models offer insights into disease progression, drug responses, and therapeutic interventions. Disease-specific tissues, such as cancer organoids, neurospheres, and cardiac tissues, recapitulate disease phenotypes and enable high-throughput screening of potential therapeutics. Artificial tissue models complement traditional animal models and accelerate the drug discovery process by providing predictive preclinical data.
Market Expansion The growing prevalence of chronic diseases, aging population, and healthcare expenditures drive the demand for artificial tissue-based therapies. Market players are investing in research and development efforts to capitalize on emerging opportunities and expand their market presence. Collaborations between academia, industry, and healthcare institutions facilitate technology transfer, knowledge exchange, and commercialization of artificial tissue products. Strategic partnerships enable the development of innovative therapies for unmet medical needs and enhance patient access to advanced regenerative treatments.
Collaboration and Partnerships Collaboration between stakeholders is essential for driving innovation and overcoming challenges in the artificial tissue market. Academic institutions, research organizations, and industry partners collaborate to advance tissue engineering technologies, develop novel biomaterials, and validate therapeutic applications. Public-private partnerships facilitate funding, infrastructure support, and regulatory guidance for artificial tissue research and development projects. Multidisciplinary collaboration fosters creativity, accelerates technology translation, and maximizes the impact of artificial tissue innovations on healthcare delivery and patient outcomes.
Ethical Considerations As artificial tissue technologies continue to advance, it's essential to address ethical considerations surrounding their development and use. Ethical frameworks help guide researchers, clinicians, and policymakers in navigating complex issues such as informed consent, privacy protection, and equitable access to healthcare. Transparency in research practices, adherence to ethical guidelines, and public engagement promote trust and accountability in artificial tissue research and clinical applications.
Global Market Expansion The artificial tissue market is not limited to developed economies but extends to emerging markets with growing healthcare needs. Market expansion efforts focus on identifying unmet medical needs, tailoring products to local healthcare contexts, and navigating regulatory requirements in diverse regions. Collaborations with local partners, knowledge-sharing initiatives, and capacity-building programs support market entry strategies and promote sustainable growth in emerging markets.
Inquire before buying, here: https://www.nextmsc.com/artificial-tissue-market/inquire-before-buying
Technological Integration Integration with other cutting-edge technologies enhances the capabilities and applications of artificial tissues in healthcare. Artificial intelligence (AI), machine learning, and data analytics tools enable data-driven insights, predictive modeling, and personalized treatment recommendations. Integration with digital health platforms, wearable devices, and telemedicine solutions facilitates remote monitoring, patient engagement, and real-time feedback for personalized healthcare delivery.
Environmental Sustainability As the artificial tissue market expands, considerations for environmental sustainability become increasingly important. Sustainable sourcing of biomaterials, energy-efficient manufacturing processes, and eco-friendly disposal practices reduce the environmental footprint of artificial tissue production. Green chemistry principles, recycling initiatives, and life cycle assessments help mitigate environmental impacts and promote responsible stewardship of natural resources in the development and utilization of artificial tissues.
By addressing these additional points, stakeholders can foster an ethical, inclusive, and sustainable ecosystem for artificial tissue innovation, ensuring its long-term viability and positive impact on healthcare and society.
Conclusion
The artificial tissue market is poised for exponential growth, fueled by technological advancements, rising healthcare needs, and increasing investment in regenerative medicine. As the field continues to evolve, stakeholders must prioritize collaboration, innovation, and regulatory compliance to realize the full potential of artificial tissues in improving patient outcomes and advancing healthcare globally. By harnessing the power of artificial tissues, researchers and clinicians can address unmet medical needs, revolutionize disease treatment paradigms, and enhance the quality of life for patients worldwide.
0 notes
Text
Toxicology Testing Services Market to Observe Strong Growth to Generate Massive Revenue in Coming Years
Latest business intelligence report released on Global Toxicology Testing Services Market, covers different industry elements and growth inclinations that helps in predicting market forecast. The report allows complete assessment of current and future scenario scaling top to bottom investigation about the market size, % share of key and emerging segment, major development, and technological advancements. Also, the statistical survey elaborates detailed commentary on changing market dynamics that includes market growth drivers, roadblocks and challenges, future opportunities, and influencing trends to better understand Toxicology Testing Services market outlook. List of Key Players Profiled in the study includes market overview, business strategies, financials, Development activities, Market Share and SWOT analysis are:
LabCorp (United States)
Charles River Laboratories (United States)
Eurofins Scientific (Luxembourg)
Bureau Veritas (France)
Envigo (United States)
Evotec AG (Germany)
Merck & Co. (United States)
SGS Group (Switzerland)
Pharmaceutical Product Development (PPD) (United States)
WuXi AppTec (China) Toxicological studies evaluate the substances to determine whether they are harmless to humans and do not change the environment. Due to the lack of robust clinical data, laboratory animal research is the most trusted way to discover the significant toxic properties of drug candidates and chemicals and to assess the potential risks to human and environmental health. Toxicological tests are the key method for determining the safety of test products that are intended to benefit humans, directly or indirectly. Toxicity test laboratories have various high-tech research facilities and experts that perform animal toxicity tests, toxicology tests, toxicology services for a range of substances, and formulated products. Safety assessment and efficacy testing are a mandatory process for industries such as chemicals, pesticides, cosmetics, consumer products, pharmaceuticals, vaccines, and medical devices. Ethical issues and pressures from animal activist groups regarding the use of animals for testing, banning animal testing of cosmetic products, helping regulators to approve in vitro tests, and discrepancies in vivo test results due to differences between species are some of the key factors in the growth of the In Vitro Toxicology Testing market. Key Market Trends: Rise in Consumer Awareness about Safety Associated With the Use of Health Care and Cosmetic Products
Increase in Concern of Toxicological Effects of Agricultural Products on Food and Environment Opportunities: Increased Funding From Governments and Research Institutes
Increased Investments in the Discovery of Newer Technologies for Toxicity Testing
The Emergence of Technologies, Such As Predictive Toxicology Testing Services Market Growth Drivers: Increase in Demand for Detection of Toxicity of Products during Their Early Stages of Development
A Rise in R&D Expenditure
The Growing Need for Newer Therapies for a Number of Conditions
Growing Preference for Outsourcing of Toxicological Studies to Contract Research Organizations Challenges: Lack of Skilled Expertise and Software The Global Toxicology Testing Services Market segments and Market Data Break Down by Type (In Vivo Method, In Vitro Method, In Silico Method), Application (Pharmaceutical & Biotechnology, Cosmetic, Chemical, Medical Devices, Others), Toxicity Type (Dermal Toxicity, Ocular Toxicity, Nephrotoxicity, Neurotoxicity, Carcinogenicity, Genotoxicity, Reproductive Toxicity, Developmental Toxicity), Technology (Cell Culture, High Throughput, Molecular Imaging, OMICS Technology)
Presented By
AMA Research & Media LLP
0 notes
Text
Global In Vitro Toxicity Testing in Chemical Market is expected to grow with a steady CAGR in the forecast by 2026

Analysis of Global In Vitro Toxicity Testing in Chemical Market report:
Global In Vitro Toxicity Testing in Chemical Market is expected to grow with a steady CAGR in the forecast period of 2019-2026. The report contains data from the base year of 2018 and the historic year of 2017. This rise in market value can be attributed to the enhanced databases of toxins and toxicology methods.
Definition:
In Vitro toxicity testing in chemical market is the testing process or method for the detection of effects of toxic substances and compounds on cells and bacteria. This method is majorly employed for the detection of any harmful chemicals or any characteristics of the chemicals that might be fatal. This method is employed during the production/development of various chemicals and chemical compounds.
Get Sample Copy of Report Here: @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-in-vitro-toxicity-testing-in-chemical-market
Market Drivers:
· Expansion of technology and innovations in services and solutions offering resulting in expanded solutions and services; this factor is expected to drive the market growth
· Expanded levels of R&D expenditure incurred by various private and governmental organisations regarding the detection and testing of toxicity; this factor is expected to drive the market growth
Market Restraints:
· Lack of adoption of the service from the various authorities and regulatory organisations; this factor is expected to act as a restraint to the market growth
· Lack of capabilities in applications and testing capability for complex processes and endpoint detection; this factor is expected to restrain the market growth
Leading Key players profiled in this report are:
Few of the major competitors currently working in in vitro toxicity testing in chemical market are Merck KGaA; Eurofins Scientific; Thermo Fisher Scientific; BioreclamationIVT; SGS SA; QIAGEN; Covance Inc.; Charles River; Gentronix; Catalent, Inc; MB Research Laboratories; GENERAL ELECTRIC COMPANY; Bio-Rad Laboratories, Inc.; Cyprotex; BioStatus Limited; Admescope Ltd; Promega Corporation and InSphero.
Global In Vitro Toxicity Testing in Chemical Market Segmentation:
GTPãS-Binding Assays
Fluorescent Imaging Plate Reader (FLIPR Assays)
G-Protein Coupled Receptors (GPCR) & Ion Channel Targets
Various Reporter-Based Assay
Eco Toxicity
Reproductive Toxicity
Developmental Toxicity
Endocrine Disruptors
Cell Culture Technologies
High-Throughput Technologies
Cellular Imaging Technologies
Toxicogenomics
Cellular Assays
Biochemical Assays
EX Vivo Models
· By Geography
o North America
o South America
o Europe
o Asia-Pacific
Company Share Analysis:
Global in vitro toxicity testing in chemical market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of in vitro toxicity testing in chemical market for global, Europe, North America, Asia-Pacific, South America and Middle East & Africa.
Get 20% Extra Discount for Early Buyer, Know More @ https://www.databridgemarketresearch.com/inquire-before-buying/?dbmr=global-in-vitro-toxicity-testing-in-chemical-market
The Global In Vitro Toxicity Testing in Chemical Market report provides the global market size of the main players in each region. Moreover, the report provides knowledge of the leading markets players within the Global In Vitro Toxicity Testing in Chemical Market. The industry changing factors for the market segments are explored in this report. This analysis report covers the growth factors of the worldwide market based on end-users. Market opportunities and recommendations for new investments are also encompassed in this report.
Get Customization and Discount on Report by emailing [email protected] . We are content with our glorious 99.9 % client satisfying rate.
Access Full Report:- https://www.databridgemarketresearch.com/reports/global-in-vitro-toxicity-testing-in-chemical-market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge Market Research provides appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Data Bridge adepts in creating satisfied clients who reckon upon our services and rely on our hard work with certitude.
Contact:
Data Bridge Market Research
+1-888-387-2818
Find More Reports Related To This Category
Global Aliphatic Solvents and Thinners Market
Global Wastewater Treatment Chemicals Market
#Global In Vitro Toxicity Testing in Chemical Market#Global In Vitro Toxicity Testing in Chemical Market trends#Global In Vitro Toxicity Testing in Chemical Market application
0 notes
Text
Global In Vitro Toxicity Testing in Chemical Market is accounted to grow with a steady CAGR in the forecast by 2026

Analysis of Global In Vitro Toxicity Testing in Chemical Market report:
Global In Vitro Toxicity Testing in Chemical Market is expected to grow with a steady CAGR in the forecast period of 2019-2026. The report contains data from the base year of 2018 and the historic year of 2017. This rise in market value can be attributed to the enhanced databases of toxins and toxicology methods.
Get Sample Copy of Report Here: @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-in-vitro-toxicity-testing-in-chemical-market
Definition:
In Vitro toxicity testing in chemical market is the testing process or method for the detection of effects of toxic substances and compounds on cells and bacteria. This method is majorly employed for the detection of any harmful chemicals or any characteristics of the chemicals that might be fatal. This method is employed during the production/development of various chemicals and chemical compounds.
Leading Key players profiled in this report are:
Few of the major competitors currently working in in vitro toxicity testing in chemical market are Merck KGaA; Eurofins Scientific; Thermo Fisher Scientific; BioreclamationIVT; SGS SA; QIAGEN; Covance Inc.; Charles River; Gentronix; Catalent, Inc; MB Research Laboratories; GENERAL ELECTRIC COMPANY; Bio-Rad Laboratories, Inc.; Cyprotex; BioStatus Limited; Admescope Ltd; Promega Corporation and InSphero.
Market Drivers:
· Expansion of technology and innovations in services and solutions offering resulting in expanded solutions and services; this factor is expected to drive the market growth
· Expanded levels of R&D expenditure incurred by various private and governmental organisations regarding the detection and testing of toxicity; this factor is expected to drive the market growth
Market Restraints:
· Lack of adoption of the service from the various authorities and regulatory organisations; this factor is expected to act as a restraint to the market growth
· Lack of capabilities in applications and testing capability for complex processes and endpoint detection; this factor is expected to restrain the market growth
Global In Vitro Toxicity Testing in Chemical Market Segmentation:
GTPãS-Binding Assays
Fluorescent Imaging Plate Reader (FLIPR Assays)
G-Protein Coupled Receptors (GPCR) & Ion Channel Targets
Various Reporter-Based Assay
Eco Toxicity
Reproductive Toxicity
Developmental Toxicity
Endocrine Disruptors
Cell Culture Technologies
High-Throughput Technologies
Cellular Imaging Technologies
Toxicogenomics
Cellular Assays
Biochemical Assays
EX Vivo Models
· By Geography
o North America
o South America
o Europe
o Asia-Pacific
The Global In Vitro Toxicity Testing in Chemical Market report provides the global market size of the main players in each region. Moreover, the report provides knowledge of the leading markets players within the Global In Vitro Toxicity Testing in Chemical Market. The industry changing factors for the market segments are explored in this report. This analysis report covers the growth factors of the worldwide market based on end-users. Market opportunities and recommendations for new investments are also encompassed in this report.
Get 20% Extra Discount for Early Buyer, Know More @ https://www.databridgemarketresearch.com/inquire-before-buying/?dbmr=global-in-vitro-toxicity-testing-in-chemical-market
Company Share Analysis:
Global in vitro toxicity testing in chemical market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of in vitro toxicity testing in chemical market for global, Europe, North America, Asia-Pacific, South America and Middle East & Africa.
Get Customization and Discount on Report by emailing [email protected] . We are content with our glorious 99.9 % client satisfying rate.
Access Full Report:- https://www.databridgemarketresearch.com/reports/global-in-vitro-toxicity-testing-in-chemical-market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge Market Research provides appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Data Bridge adepts in creating satisfied clients who reckon upon our services and rely on our hard work with certitude.
Contact:
Data Bridge Market Research
+1-888-387-2818
Find More Reports Related To This Category
Global Aliphatic Solvents and Thinners Market
Global Wastewater Treatment Chemicals Market
#Global In Vitro Toxicity Testing in Chemical Market#Global In Vitro Toxicity Testing in Chemical Market trends#Global In Vitro Toxicity Testing in Chemical Market overview#Global In Vitro Toxicity Testing in Chemical Market applications
0 notes
Text
Global In Vitro Toxicity Testing in Chemical Market is expected to grow with a steady CAGR in the forecast by 2026

Definition:
In Vitro toxicity testing in chemical market is the testing process or method for the detection of effects of toxic substances and compounds on cells and bacteria. This method is majorly employed for the detection of any harmful chemicals or any characteristics of the chemicals that might be fatal. This method is employed during the production/development of various chemicals and chemical compounds.
Analysis of Global In Vitro Toxicity Testing in Chemical Market report:
Global In Vitro Toxicity Testing in Chemical Market is expected to grow with a steady CAGR in the forecast period of 2019-2026. The report contains data from the base year of 2018 and the historic year of 2017. This rise in market value can be attributed to the enhanced databases of toxins and toxicology methods.
Get Sample Copy of Report Here: @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-in-vitro-toxicity-testing-in-chemical-market
Market Drivers:
· Expansion of technology and innovations in services and solutions offering resulting in expanded solutions and services; this factor is expected to drive the market growth
· Expanded levels of R&D expenditure incurred by various private and governmental organisations regarding the detection and testing of toxicity; this factor is expected to drive the market growth
Market Restraints:
· Lack of adoption of the service from the various authorities and regulatory organisations; this factor is expected to act as a restraint to the market growth
· Lack of capabilities in applications and testing capability for complex processes and endpoint detection; this factor is expected to restrain the market growth
Leading Key players profiled in this report are:
Few of the major competitors currently working in in vitro toxicity testing in chemical market are Merck KGaA; Eurofins Scientific; Thermo Fisher Scientific; BioreclamationIVT; SGS SA; QIAGEN; Covance Inc.; Charles River; Gentronix; Catalent, Inc; MB Research Laboratories; GENERAL ELECTRIC COMPANY; Bio-Rad Laboratories, Inc.; Cyprotex; BioStatus Limited; Admescope Ltd; Promega Corporation and InSphero.
Global In Vitro Toxicity Testing in Chemical Market Segmentation:
GTPãS-Binding Assays
Fluorescent Imaging Plate Reader (FLIPR Assays)
G-Protein Coupled Receptors (GPCR) & Ion Channel Targets
Various Reporter-Based Assay
Eco Toxicity
Reproductive Toxicity
Developmental Toxicity
Endocrine Disruptors
Cell Culture Technologies
High-Throughput Technologies
Cellular Imaging Technologies
Toxicogenomics
Cellular Assays
Biochemical Assays
EX Vivo Models
· By Geography
o North America
o South America
o Europe
o Asia-Pacific
Company Share Analysis:
Global in vitro toxicity testing in chemical market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of in vitro toxicity testing in chemical market for global, Europe, North America, Asia-Pacific, South America and Middle East & Africa.
Get 20% Extra Discount for Early Buyer, Know More @ https://www.databridgemarketresearch.com/inquire-before-buying/?dbmr=global-in-vitro-toxicity-testing-in-chemical-market
The Global In Vitro Toxicity Testing in Chemical Market report provides the global market size of the main players in each region. Moreover, the report provides knowledge of the leading markets players within the Global In Vitro Toxicity Testing in Chemical Market. The industry changing factors for the market segments are explored in this report. This analysis report covers the growth factors of the worldwide market based on end-users. Market opportunities and recommendations for new investments are also encompassed in this report.
Get Customization and Discount on Report by emailing [email protected] . We are content with our glorious 99.9 % client satisfying rate.
Access Full Report:- https://www.databridgemarketresearch.com/reports/global-in-vitro-toxicity-testing-in-chemical-market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge Market Research provides appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Data Bridge adepts in creating satisfied clients who reckon upon our services and rely on our hard work with certitude.
Contact:
Data Bridge Market Research
+1-888-387-2818
Find More Reports Related To This Category
Global Aliphatic Solvents and Thinners Market
Global Wastewater Treatment Chemicals Market
#Global In Vitro Toxicity Testing in Chemical Market#Global In Vitro Toxicity Testing in Chemical Market trends#Global In Vitro Toxicity Testing in Chemical Market applications#Global In Vitro Toxicity Testing in Chemical Market overview
0 notes
Text
Pharmaceutical and Biotechnology Lead to Toxicology Testing Market Growth
Pharmaceutical and Biotechnology Lead to Toxicology Testing Market Growth
Pharmaceuticals and Biotechnology industries are heavily relying on toxicology testing market. There is a constant need for toxicology testing market research reports for the development of effective toxicology testing methodologies and for regulatory conformity. They also use this market research to overcome potential threats and to better understand the toxicology process and its applications. Because of its importance, toxicology testing market is witnessing explosive growth right now. In this report, we discuss the toxicology testing market players.
Global Toxicology Testing Market: Global toxicology testing market is witnessing exponential growth due to increasing demands from the global market for reliable toxicology testing product and methodologies. This toxicology testing market is witnessing intense competition due to the emergence of new toxicology testing products in the global market. There are intense growth potential and a major share of this toxicology testing market in Asia, Middle East and North America. Asia has the largest number of toxicology testing product manufacturers. The top five toxicology testing product manufacturers in Asia are Korea Biotechnology Co Ltd, Dow Chemical Co, Global Residences Inc., Novartec Corporation, Oceanic Corporation and Elestar.
Global toxicology testing market: As indicated by the above scenario, there is an increasing demand for toxicology testing product and methodologies from the global toxicology testing market. The key drivers behind this demand are: (a) increased safety standards of products and processes used by regulatory agencies and (b) the need to provide accurate toxicology findings and post toxicology testing for regulatory approval. Safety assessment is required for many industries, including cosmetics, personal care products, pharmaceuticals, water treatment and distribution, pulp and paper industry, automotive, packaging and food processing. In addition, the toxicology testing market can be channelized according to the level of toxicity of the product, whether it is a health hazard environmental toxicology concern or a food toxicology concern. For example, safety assessment is required for cosmetics that contain sunscreen agents, which can cause skin cancer and birth defects; for toys that contain lead or toxic metals that can adversely affect development of the nervous system; and for pharmaceuticals that contain toxic compounds that can cause nerve damage and neurological disorders.
Drug Discovery/ toxicology testing: According to the aforementioned scenario, the toxicology testing market growth in the USA has significantly raised due to the increased toxicology testing requirements of various industries. The growth potential is attributed to the fact that drug discovery requires biotechnology strategies to identify toxic drugs during the early clinical stage. The biotechnology tools and technologies are highly costly. Due to this, toxicology testing is a cost-effective toxicology testing methodology that can be utilized by toxicology laboratories or drug discovery companies to identify toxic drugs in their initial or clinical stage of development. Toxins can also be identified using advanced genetic profiling techniques.
Safety Assessment: toxicology testing can also be utilized in the safety assessment. In toxicology safety assessment, toxicology consultants perform a series of laboratory investigations that can provide toxicology experts toxicology guidance that can help them predict the toxicology outcome of a new drug that has not been subjected to the primary toxicology testing process. Based on the predicted toxicology outcomes, the toxicology consultant can help the safety analysis phase. After safety assessments, the toxicology consultant can create an exposure plan that will control toxic exposure if toxicology testing has been performed and predicted toxicities have been identified.
Market Size and Growth: Although in-vitro toxicology testing market is just one of the many markets available within the toxicology field, this toxicology testing market forecast period segmentation provides insight into the future scope of this toxicology market. As a result of this market forecast period segmentation, it is anticipated that this toxicology market will continue to expand upwards in the years to come. This toxicology testing market is expected to grow as the demand for advanced drug designs increase in the next few years. Currently, there are approximately sixteen companies in this toxicology testing market. In addition to the competitors, there are many small to mid-sized companies that have recently entered the toxicology testing market.
Revenue forecasts for this toxicology testing market is dependent on the amount of money that toxicology testing firms spend on research and development. The larger the research and development expenditures of toxicology testing firms the more money this industry will generate revenue in the future. One thing is for sure, the US drugs alerts market will continue to expand as there will be a large number of drugs that will become toxic. As long as there will be toxicology testing firms in this market, there will also be toxicology testing businesses. The forecast for revenues generated from this market is excellent provided that toxicology testing firms continue to invest and provide quality service to their customers.
Other key business sectors that can contribute to toxicology testing market growth include pharmaceuticals, bio-tech, health care, cosmetic manufacturers, nutritional supplement manufacturers, environmental remediation and waste management. The toxicology testing market can also become a main seller in the pharmaceutical industry as more toxicology assays are adopted by biotechnology industries. There are a lot of other chemicals that can be tested for toxicology purposes but none of these tests are very comprehensive as they don't allow for the complete molecular makeup of toxic substances. However, there are some toxicology testing assays that are used in drug development that can generate comprehensive toxicology testing results. With the growth of toxicology testing, a clearer picture of toxicology will be developed thus leading to more accurate toxicology testing.
Summary According to XYZResearch study, over the next 5 years the Cloud Access Security Broker (CASB) Software market will register a xx% CAGR in terms of revenue, the global market size will reach xx Million USD by 2026, from xx Million USD in 2020. In particular, It should be noted that the impact of the epidemic has accelerated the trend of localization, regionalization and decentralization of the global industrial chain and supply chain, so it is inevitable to reconstruct the global industrial chain. Faced with the global industrial change in the post epidemic era, enterprises in various countries must take precautions. This report presents revenue, market share and growth rate for each key company. In this analysis report, we will find below details: 1. Full in-depth analysis of the market structure along with forecast from 2021 to 2026 of the various segments of the Global Cloud Access Security Broker (CASB) Software market. 2. Who is the leading company in Cloud Access Security Broker (CASB) Software market, competitive analysis of key companies, mergers and acquisitions, market dynamics. 3. Which region has become the biggest growth area in Cloud Access Security Broker (CASB) Software market? 4. The Most Potential segment in each regional market. 5. Insights about factors affecting the market growth, including the impact of COVID -19. 6. Global Cloud Access Security Broker (CASB) Software market based on value chain analysis, and SWOT analysis. 7. Regional market analysis to the current revenue (Million USD) and future prospective. Major players operating in Cloud Access Security Broker (CASB) Software market-Competitive Analysis: Netskope Microsoft Oracle Cloudlock IBM Symantec Trend Micro Palo Alto Networks,Inc Skyhigh Networks Bitglass Perimeter 81 Zscaler CipherCloud Regional Segmentation (Value; Revenue, USD Million, 2015 - 2026) of Cloud Access Security Broker (CASB) Software Market by XYZResearch include: China EU USA Japan India Southeast Asia South America Type Outlook (Value; Revenue, USD Million, 2015 - 2026): SaaS PaaS IaaS Other Application Outlook (Value; Revenue, USD Million, Market Share, 2015 - 2026): BFSI Industrial Controlling Systems Automotive Retail Education Healthcare Service Providers Other
For more details contact as https://www.reportmines.com/contact-us.php
1 note
·
View note
Text
Global In Vitro Toxicity Testing in Chemical Market is expected to grow with a steady CAGR in the forecast by 2026
Definition:
In Vitro toxicity testing in chemical market is the testing process or method for the detection of effects of toxic substances and compounds on cells and bacteria. This method is majorly employed for the detection of any harmful chemicals or any characteristics of the chemicals that might be fatal. This method is employed during the production/development of various chemicals and chemical compounds.
Analysis of Global In Vitro Toxicity Testing in Chemical Market report:
Global In Vitro Toxicity Testing in Chemical Market
is expected to grow with a steady CAGR in the forecast period of 2019-2026. The report contains data from the base year of 2018 and the historic year of 2017. This rise in market value can be attributed to the enhanced databases of toxins and toxicology methods.
Get Sample Copy of Report Here: @
https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-in-vitro-toxicity-testing-in-chemical-market
Market Drivers:
· Expansion of technology and innovations in services and solutions offering resulting in expanded solutions and services; this factor is expected to drive the market growth· Expanded levels of R&D expenditure incurred by various private and governmental organisations regarding the detection and testing of toxicity; this factor is expected to drive the market growth
Market Restraints:
· Lack of adoption of the service from the various authorities and regulatory organisations; this factor is expected to act as a restraint to the market growth· Lack of capabilities in applications and testing capability for complex processes and endpoint detection; this factor is expected to restrain the market growth
Leading Key players profiled in this report are:
Few of the major competitors currently working in in vitro toxicity testing in chemical market are Merck KGaA; Eurofins Scientific; Thermo Fisher Scientific; BioreclamationIVT; SGS SA; QIAGEN; Covance Inc.; Charles River; Gentronix; Catalent, Inc; MB Research Laboratories; GENERAL ELECTRIC COMPANY; Bio-Rad Laboratories, Inc.; Cyprotex; BioStatus Limited; Admescope Ltd; Promega Corporation and InSphero.
Global In Vitro Toxicity Testing in Chemical Market Segmentation:
GTPãS-Binding Assays
Fluorescent Imaging Plate Reader (FLIPR Assays)
G-Protein Coupled Receptors (GPCR) & Ion Channel Targets
Various Reporter-Based Assay
Eco Toxicity
Reproductive Toxicity
Developmental Toxicity
Endocrine Disruptors
Cell Culture Technologies
High-Throughput Technologies
Cellular Imaging Technologies
Toxicogenomics
Cellular Assays
Biochemical Assays
EX Vivo Models
·
By Geography
o North Americao South Americao Europeo Asia-Pacific
Company Share Analysis:
Global in vitro toxicity testing in chemical market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of in vitro toxicity testing in chemical market for global, Europe, North America, Asia-Pacific, South America and Middle East & Africa.
Get 20% Extra Discount for Early Buyer, Know More @
https://www.databridgemarketresearch.com/inquire-before-buying/?dbmr=global-in-vitro-toxicity-testing-in-chemical-market
The Global In Vitro Toxicity Testing in Chemical Market report provides the global market size of the main players in each region. Moreover, the report provides knowledge of the leading markets players within the Global In Vitro Toxicity Testing in Chemical Market. The industry changing factors for the market segments are explored in this report. This analysis report covers the growth factors of the worldwide market based on end-users. Market opportunities and recommendations for new investments are also encompassed in this report. Get
Customization
and
Discount
on Report by emailing
. We are content with our glorious 99.9 % client satisfying rate.
Access Full Report:- https://www.databridgemarketresearch.com/reports/global-in-vitro-toxicity-testing-in-chemical-market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge Market Research provides appropriate solutions to the complex business challenges and initiates an effortless decision-making process.Data Bridge adepts in creating
satisfied
clients
who reckon upon our services and rely on our hard work with
certitude
.
Contact:
Data Bridge Market Research+1-888-387-2818
Find More Reports Related To This Category
Global Aliphatic Solvents and Thinners Market
Global Wastewater Treatment Chemicals Market
#Global In Vitro Toxicity Testing in Chemical Market#Global In Vitro Toxicity Testing in Chemical Market trends#Global In Vitro Toxicity Testing in Chemical Market application#Global In Vitro Toxicity Testing in Chemical Market size
0 notes
Text
Medical Device Testing Market Industry Share, Size, Growth, Demands, Revenue, Top Leaders and Forecast to 2029
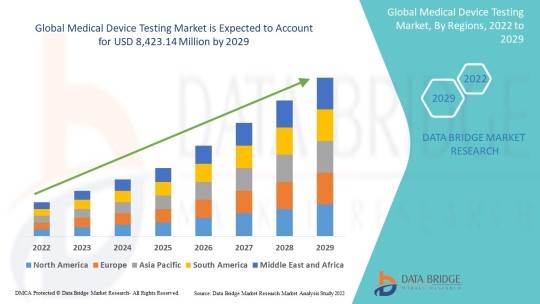
Industry Analysis
Medical device testing market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 10.8% in the forecast period of 2022 to 2029 and is expected to reach USD 8,423.14 million by 2029 from USD 3,832.27 million in 2021.
Additionally, the credible Medical Device Testing Market report helps the manufacturer in finding out the effectiveness of the existing channels of distribution, advertising programs, or media, selling methods and the best way of distributing the goods to the eventual consumers. Taking up such market research report is all the time beneficial for any company whether it is a small scale or large scale, for marketing of products or services. It makes effortless for healthcare industry to visualize what is already available in the market, what market anticipates, the competitive environment, and what should be done to surpass the competitor.
Get a Free Sample of The Report: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-medical-device-testing-market
Market Insights and Scope
Medical device testing is the process of demonstrating that the device is reliably and safely perform in use. In new product development, extensive design validation testing is applied. This includes performance testing, toxicity and chemical analysis, and sometimes human factors or even clinical testing. Ongoing quality assurance testing is generally more limited. This usually include dimensional checks, some functional tests, and packaging verification. Various types of medical testing services are available there in the market such as inspection services, certification services and among others.
The Medical Device Testing Market report encompasses various segments linked to healthcare industry and market with comprehensive research and analysis. These comprise industry outlook with respect to critical success factors (CSFs), industry dynamics that mainly covers drivers and restraints, market segmentation & value chain analysis, key opportunities, application and technology outlook, regional or geographical insight, country-level analysis, key company profiles, competitive landscape, and company market share analysis. All the data, figures and information are backed up by well recognized analysis tools which include SWOT analysis and Porter’s Five Forces analysis. So, take business to the peak level of growth with the all-inclusive Data Bridge Market research report.
Get full access to the report: https://www.databridgemarketresearch.com/reports/global-medical-device-testing-market
Industry Segmentation and Size
Global medical device testing market is segmented into service type, testing type, phase, sourcing type, device class and product. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Service Type
Testing services
Inspection services
Certification services
Testing Type
Physical testing
Chemical/biological testing
Cybersecurity testing
Microbiology and sterility testing
Others
Phase
Preclinical
Clinical
Sourcing Type
Outsourced
In-house
Device Class
Class I
Class II
Class III
Product
Active implant medical device
Active medical device
Non-active medical device
In-vitro diagnostics medical device
Opthalmic medical device
Orthopedic and dental medical device
Vascular medical device
Others
Market Country Level Analysis
The countries covered for this market are
U.S., Canada, Mexico, Germany, France, U.K., Italy, Spain, Netherlands, Russia, Switzerland, Turkey, Belgium, Rest of Europe, China, Japan, India, South Korea, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa.
A reliable Medical Device Testing Market marketing report proves to be the finest and excellent market research report as it is formulated with the following critical factors. These consist of primary research, benchmarking studies, secondary research, company profiles, competitive intelligence & reporting, syndicated research, data collection, data processing and analysis, survey design, and survey programming. The report performs market study and analysis to provide market data by considering new product development from beginning to launch. The healthcare business report also provides evaluations based on the market type, organization size, availability on-premises, end-users’ organization type, and the availability in areas such as North America, South America, Europe, Asia-Pacific and Middle East & Africa.
Industry Share Analysis
Some of the major players operating in the medical device testing market are
Intertek Group plc, SGS SA, Bureau Veritas, TUV SUD, TUV Rheinland, Pace, Charles River Laboratories, Biomedical Device Labs, UL LLC, North American Science Associates, LLC, Medistri SA, WuXi AppTec, NSF, Labcorp, Eurofins Scientific, Nelson Laboratories, LLC- A Sotera Health company, Gateway Analytical, ITC ZLIN, Element Materials Technology, EndoLab Mechanical Engineering GmbH, Hohenstein, Medical Engineering Technologies Ltd., Bioneeds, Cigniti, Arbro Pharmaceuticals Private Limited & Auriga Research Private Limited, Q Laboratories, IMR Test Labs among others.
Browse Related Reports@
Global Whiskey Market
South Africa Battery Market
Global Plant-Based Egg Market
Global Nutritional Beverages market
MENA Tahini market
Global Dental Membrane and Bone Graft Substitute Market
About Us: Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact: Data Bridge Market Research Tel: +1-888-387-2818 Email: [email protected]
#Medical Device Testing Market Growing Popularity#Medical Device Testing Market Global Leading Brands#Medical Device Testing Market drivers-advantages#Medical Device Testing Market Segmentation-CAGR rate#Medical Device Testing Market Demands-Size-Share-Top Trends#Medical Device Testing Market Industry-Competitors#Medical Device Testing Market Growth-Competition#Medical Device Testing Market 2029 by Types-Application#Medical Device Testing Market Healthcare Industry
0 notes
Text
In-vitro Toxicology Testing Market overview by recent opportunities, growth size, regional analysis and forecasts to 2031 | General Electric Company, Thermo Fisher Scientific In., Laboratory Corporation of America Holdings
Global In-vitro Toxicology Testing Market report from Global Insight Services is the single authoritative source of intelligence on In-vitro Toxicology Testing Market. The report will provide you with analysis of impact of latest market disruptions such as Russia-Ukraine war and Covid-19 on the market. Report provides qualitative analysis of the market using various frameworks such as Porters’ and PESTLE analysis. Report includes in-depth segmentation and market size data by categories, product types, applications, and geographies. Report also includes comprehensive analysis of key issues, trends and drivers, restraints and challenges, competitive landscape, as well as recent events such as M&A activities in the market.
In-vitro toxicology testing is a type of toxicology testing that is conducted using cells or other tissues that are cultured in a laboratory. This type of testing is used to assess the potential toxicity of a substance, and to determine how a substance may affect the function of cells or tissues. In-vitro toxicology testing is often used in the development of new drugs or chemicals, and in the assessment of environmental exposures.
Request Sample Report – https://www.globalinsightservices.com/request-sample/GIS21207/
Key Trends
One of the key trends in in-vitro toxicology testing is the use of new technologies to assess the toxicity of substances. In particular, there is a growing interest in the use of 3D cell culture systems for in-vitro toxicology testing.
Another key trend is the use of alternative methods to assess the toxicity of substances. In particular, there is a growing interest in the use of in-silico methods, which use computer models to predict the toxicity of substances.
Key Drivers
There are several key drivers of the in-vitro toxicology testing market.
Firstly, there is a growing awareness of the ethical concerns associated with animal testing, and an increasing number of consumers are demanding that companies switch to more humane testing methods.
Secondly, in-vitro testing is generally more accurate than animal testing, as it can more accurately replicate the human cellular environment.
Market Segmentation
By Type
Absorption
Toxic Substances
By Technology
Cell Culture Technologies
Toxicogenomics
By End-Use
Cosmetics
Pharmaceuticals
By Region
North AmericaUS
Free Customization Available – https://www.globalinsightservices.com/request-customization/GIS21207
Key Players
General Electric Company
Thermo Fisher Scientific In.
Laboratory Corporation of America Holdings
Acacia Pharma Group Plc
Tesaro Inc
Helsinn Holding S.A.
Heron Therapeutics Inc
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with lead analyst of the report
Excel data pack included with all report purchases
Robust and transparent research methodology
Ground breaking research and market player-centric solutions for the upcoming decade according to the present market scenario
About Global Insight Services:
Global Insight Services (GIS) is a leading multi-industry market research firm headquartered in Delaware, US. We are committed to providing our clients with highest quality data, analysis, and tools to meet all their market research needs. With GIS, you can be assured of the quality of the deliverables, robust & transparent research methodology, and superior service.
Contact Us:
Global Insight Services LLC
16192, Coastal Highway, Lewes DE 19958
E-mail: [email protected]
Phone: +1–833–761–1700
0 notes
Text
Global Organ-on-a-chip Market Size, Share Report, 2030
Organ-on-a-chip Market Overview:
With escalating demand for advanced organ-on-chip devices by the healthcare sector, it is presumed that the market size will continue to expand rapidly in the coming years. Organ-on-chip devices are used primarily in in-vitro assessment of biochemicals, real-time imaging, and metabolic and genetic activities of the living cells present in a functional tissue. The steadily expanding application scope of OOCs is expected to boost the market demand in the years to come.
Drug screening is an effective and cost-effective technique that is deployed for rapidly assessing samples. Researchers and scientists make use of organ-on-chip culture devices to evaluate the effects of different drugs within the body. In addition, drug toxicity or effectiveness of the drug in different organs is assessed using this method, and the rising number of these processes is expected to favor the global market. It is believed that lower number of donors for lungs to be transplanted is one of the leading causes for patient deaths. This has resulted in a significant need to design functional, laboratory-engineered organs, which ultimately bolsters the Organ-on-a-chip market growth.
The biopharmaceutical sector and the research community have become extremely proactive since the COVID-19 outbreak. Following the pandemic, organ-on-chip has turned into one of the most versatile technologies used for developing drugs and vaccines. Simply put, the novel coronavirus has emerged as a lucrative opportunity for the organ-on-a-chip market and will ensure consistent growth throughout the evaluation period.
Organ-on-a-chip Market Segmentation:
The organ-on-a-chip industry has been studied with respect to organ type, application, and end-user.
The various organ types considered in the report include heart-on-chip, lung-on-chip, intestine-on-chip, liver-on-chip, skin-on-chip, kidney-on-chip, human-on-chip, blood-brain barrier-on-chip, artificial organ companies and others.
Top applications of OOCs are toxicology research, drug discovery, and more. Drug discovery is projected to be the top segment and would therefore, capture the highest portion of the global market. OOCs are extensively deployed by pharmaceutical firms for drug discovery as well as clinical trials for diverse chronic therapeutic areas. The second position has been taken by the toxicology research segment, thanks to the surge in R&D activities, government efforts along with funding given to the research institutes, and the universities focused on using OOCs during toxicology research as well as drug testing.
Organ-on-a-chip Market Regional Analysis:
The five top geographies considered in the market report include Europe, Latin America, APAC/Asia Pacific along with the Middle East & Africa/MEA, and North America.
America as emerged as the market leader, largely due to the wide availability of extensive lines of services provided by the renowned players and the surge in chemicals’ toxicological testing on a variety of organ cells. Massive spending by private as well as public institutes on exhaustive research activities also add to the market worth in the region. A large number of research institutions in the region are proactively carrying out research activities and studies to accelerate technologies for organ-on-chip, which could mean further market elevation in the future.
Europe is placed second in the global industry, and it is also presumed that the region will continue to thrive at a healthy rate between 2020 and 2027. Establishment of numerous reputed OOC developers across Germany, the UK, Italy, Spain, and France, along with government efforts as well as funding for research activities benefit the European market. The rise in collaborations between companies and institutes and universities for the development of better quality OOCs, coupled with the presence of a well-established healthcare infrastructure will also foster the market size in the following years.
Asia Pacific is touted to capture the fastest growth rate in the years ahead as the region has a favorable regulatory framework that has fostered the approval rate of OOCs in Australia, South Korea, China, and Japan. Also, the high burden of various chronic diseases, extensive use of OOC devices by the healthcare industry and the soaring demand in drug screening will add to the market value in the years to come. Apart from this, the rising OOC demand for kidney and lung -based organ culture, and the incredible increase in the number of research activities pertaining to OOC devices could leave a positive impact on the regional market as well.
Organ-on-a-chip Market Players:
CN Bio Innovations Limited (UK)
Emulate Inc. (US)
TissUse GmbH (Germany)
MIMETAS BV (Netherlands)
Hµrel Corporation
Nortis Inc. (US)
InSphero (Switzerland)
TARA Biosystems Inc. (US)
Axosim (US)
Organovo Holdings Inc. (US)
BioIVT (US)
HemoShear Therapeutics
Among others are some key companies in the global Organ-on-a-chip that are listed by MRFR for market research.
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), & Consulting Services. MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients.
Contact us:
Market Research Future (part of Wantstats Research and Media Private Limited),
99 Hudson Street, 5Th Floor,
New York, New York 10013
United States of America
+1 628 258 0071
Email: [email protected]
0 notes
Text
In-vitro Toxicology Testing Market Expected To Cross $51.1 Billion By 2028
The global in-vitro toxicology testing market size is expected to reach USD 51.1 billion by 2028, according to a new report by Grand View Research, Inc. The market is expected to expand at a CAGR of 10.7% from 2021 to 2028. In recent times, the validation and acceptance of in-vitro and alternative testing methods by regulatory agencies are increasing at lucrative pace. Also, ongoing technological advancements to replace the use of animals for toxicology testing purposes have spurred the use of in vitro testing models, in turn, driving the market.
Several governments are taking measures to minimize animal-based test models, forming conducive government policies, and providing funds to support in-vitro models. These factors are expected to create ample growth opportunities for the market. For instance, in November 2019, the U.S. National Institute for Environmental Health Sciences planned to provide funds to small companies for the development of engineered 3D culture or organotypic culture models (OCM) in-vitro systems.
With advancements in high throughput screening, biological screening, and chemical synthesis, the number of publicly available databases containing data related to toxicity; absorption, distribution, metabolism, and excretion (ADME); pharmacovigilance; and drug screening has expanded rapidly. This has enabled scientists to access vast information for toxicity profiling, thereby spurring revenue generation in this market. Several companies offer HTT-based in vitro toxicology analysis, e.g., Solidus Bioscience’s MetaChip Technology, which provides a solution for in vitro toxicology analysis using HTT.
Request a free sample copy or view report summary: In-vitro Toxicology Testing Market Report
In-vitro Toxicology Testing Market Report Highlights
Growing application of 3D-spheroid-cultures, particularly for nanoparticle toxicity testing, resulted in the dominance of the cell culture technology segment in 2020
Omics technology is anticipated to register lucrative growth over the forecast period as RNA sequencing and shotgun proteomics are increasingly used in research settings
The cosmetics industry is expected to be the fastest-growing segment in the near future, creating new avenues for the expansion of HTTs across the globe
The cellular assays segment dominated the market in 2020 owing to advancements in cell-based technologies, including label-free detection and high-content screening
The potential of cell imaging technologies is combined with high-content cell screening assays and high-throughput assays for the development of multi-parameter assays
Systemic toxicology emerged as the leading application segment in 2020 as it plays a key role in risk assessment during drug development procedures
For instance, toxicity testing of systemic immunosuppressive drugs and systemic corticosteroids is important during the development of drugs for ocular inflammatory disease
The pharmaceutical industry segment dominated the market in 2020 as toxicological testing is a prerequisite step for drug development
Stringent government regulations regarding animal usage for toxicity analysis in North America have led to an increased adoption rate of in-vitro models
In Asia Pacific, the market is anticipated to witness the fastest growth rate over the forecast period
This is due to increasing initiatives by public agencies to encourage acceptance of non-animal test models
The key participants are involved in collaborations with bioinformatics research firms in order to develop novel in-silico algorithms for computational estimation of toxicity of pharmaceutical and chemical products
Moreover, these market entities are also focusing on entering untapped regions in order to maintain their share in market revenue
In-vitro Toxicology Testing Market Segmentation
Grand View Research has segmented the global in-vitro toxicology testing market on the basis of end-use, technology, method, application, and region:
In-vitro Toxicology Testing End-use Outlook, (Revenue, USD Million, 2018 - 2028)
Pharmaceutical Industry
Cosmetics & Household Products
Academic Institutes and Research Laboratories
Diagnostics
Chemical Industry
Food Industry
In-vitro Toxicology Testing Technology Outlook (Revenue, USD Million, 2018 - 2028)
Cell Culture Technology
High Throughput Technology
Molecular Imaging
OMICS Technology
In-vitro Toxicology Testing Application Outlook, (Revenue, USD Million, 2018 - 2028)
Systemic Toxicology
Dermal Toxicity
Endocrine Disruption
Occular Toxicity
Others
In-vitro Toxicology Testing Method Outlook (Revenue, USD Million, 2018 - 2028)
Cellular Assay
Biochemical Assay
In-Silico
Ex-vivo
Live Cells
Fixed Cells
High Throughput / High Content Screening
Molecular Imaging
Others
Confocal Microscopy
Others
In-vitro Toxicology Testing Regional Outlook (Revenue, USD Million, 2018 - 2028)
North America
Europe
Asia Pacific
Latin America
Middle East Africa (MEA)
U.S.
Canada
Germany
U.K.
Japan
China
Brazil
South Africa
List of Key Players of In-vitro Toxicology Testing Market
Merck KGaA
Charles River
Bio-Rad Laboratories, Inc.
Abbott
Thermo Fisher Scientific Inc.
Catalent, Inc.
GE Healthcare
Quest Diagnostics Incorporated
Eurofins Scientific
Laboratory Corporation of America Holdings
Evotec
Creative Bioarray
Gentronix
BioIVT
SGS SA
Agilent Technologies.
About Grand View Research
Grand View Research, Inc. is a U.S. based market research and consulting company, registered in the State of California and headquartered in San Francisco. The company provides syndicated research reports, customized research reports, and consulting services. To help clients make informed business decisions, we offer market intelligence studies ensuring relevant and fact-based research across a range of industries, from technology to chemicals, materials and healthcare.
0 notes
Text
Global Contract Research Organization (CRO) Services Market Analysis, Application and Forecast to 2021-2026
Bharat Book Bureau Provides the Trending Market Research Report on “Global Markets for Contract Research Organization (CRO) Services”under Life Sciences Market Research Reports Category. The report offers a collection of superior market research, market analysis, competitive intelligence and Market reports.
The Global CRO Market is identified in this report, along with all major global CRO companies. The total CRO market is broken out globally and by geographic region. All major therapeutic areas covered by CROs in the clinical trial process are detailed.
The service areas provided by CROs are extensively covered, as well. The phases of the clinical trial process and the role provided by CROs are detailed in this report, as well. The current report will provide detailed examination of CRO services, analyzing market trends for CRO services with data from 2020, estimates from 2021 and projections of CAGR through 2026 (forecast period 2021-2026).
This report provides detailed analysis of the top CROs and evolving roles in the clinical trial process. Faced with the rapid development of new therapies, pharmaceutical companies increasingly turn to CROs.
CRO expertise within specific therapeutic areas and experience with new and adaptive study design protocols, can reduce costs and study duration. With typical clinical trials costing nearly $2.5 billion and spanning over a decade, from initial testing to Phase IV and post market approval, a thriving market exists to lower these costs and timelines.
CROs are expected to continue to play an increasing role in many facets of the clinical trial process. Oncology, neurology, cardiovascular, metabolism-diabetes, vaccines and other rapidly growing therapeutic areas that have a significant number of drugs in the clinical trial process are detailed in this report.
Strong demand for CROs leads to heightened market valuations and to a plethora of blockbuster mergers and acquisitions (M&As). M&As led to a few powerful companies with expertise across many areas of the clinical trial process. Niche players are still playing a critical role. Emerging trends and changing dynamics within the CRO industry are analyzed, in detail, in this report.
Report Includes:
35 tables
An updated review of the global markets for in vitro toxicity testing and technologies under development
Analyses of the global market trends, with data from 2019 to 2020, estimates for 2021, and projections of compound annual growth rates (CAGRs) through 2026
Technology assessment of the key drivers, restraints and opportunities that will shape the market for in vitro toxicity testing over the next five years (2021 to 2026)
Evaluation and forecast the overall market size, and corresponding market share analysis by testing method, component, application, technology, end-user industry, and geographic region
Highlights of the impact of COVID-19 on the progress of this market
Review of key technology developments, latest market trends, and other influential factors such as validation and testing strategies for pharmaceuticals, cosmetics, and chemicals
Insight into recent industry structure, current competitive scenario, R&D activities, and regulatory and legislative issues currently focused on in vitro toxicology
Descriptive company profiles of the leading market players including Charles River Laboratories, Evotec, Frontage Labs, ICON PLC, Medpace, PPD Inc., and Syneos Health
Browse our full report with Table of Content : https://www.bharatbook.com/report/1113396/global-market-for-contract-research-organization-cro-services
About Bharat Book Bureau: Bharat Book is Your One-Stop-Shop with an exhaustive coverage of 4,80,000 reports and insights that includes latest Market Study, Market Trends & Analysis, Forecasts Customized Intelligence, Newsletters and Online Databases. Overall a comprehensive coverage of major industries with a further segmentation of 100+ subsectors.
Contact us at: Bharat Book Bureau Tel: +91 22 27810772 / 27810773 Email: [email protected] Website: www.bharatbook.com
0 notes